I've seen people around the Internet referring to their plate configurations using a particular method that seems to make sense.
So for those of you that aren't knowledgeable on this yet I'll attempt to explain it.
UPDATED: 29 July, 2008
UPDATED AGAIN: 9 August, 2008
General Concepts
For this section all plates, pipes, strips, rods, springs or any other shape that is used to electrolyze water is an electrode.
An electrode that is connected directly to the positive lead is designated with a plus sign (+).

An electrode that is connected directly to the negative lead is designated with a minus sign (-).

Electrodes that are not connected to either positive or negative leads, but are placed between them are both positive on one side and negative on the other. This is due to current jumping from the positive electrode to the negative surface of the unconnected electrode, then flowing through the electrode to the other side where it jumps off what is now the positive side of the electrode to the negative side of the adjoining electrode. These electrodes are referred to as neutral plates and are designated with the letter n.

Whenever three or more neutral electrodes are together in a design, the number of 'n's can be replaced by the actual number of adjacent neutral electrodes followed by an n. For example, there is a 31 plate design floating around the Internet and to use this example it would have 29 neutral electrodes in its configuration. This particular design would be written as such: +29n-.
For assemblies that use isolated cells use square braces [] to designate series configuration and parentheses {} to indicate parallel configuration.
Plate Assemblies
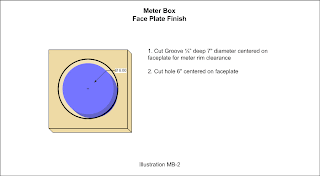
Plate assemblies can be flat, conical or bowled in shape, but generally flat and made from sheet metal. Plate assemblies are the dominant form of electrode so a designation normally is omitted. However, if needed the letter P may be used to designate plates like so: +P- If a plate assembly is conical, then the C modifier would be used like so: +CP- Likewise if a plate assembly is bowled, then the B modifier is used like so: +BP-
A really simple plate assembly with just one positive plate and one negative plate would have the nomenclature +-.
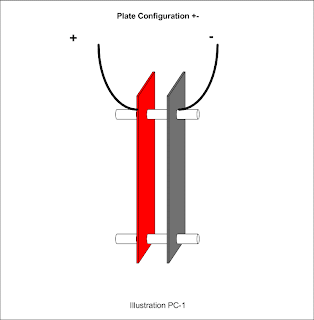
A slightly more complicated plate assembly with a neutral plate in the middle would be designated +n-.

A third example, where 6 plates alternate between positive and negative would have the following nomenclature: +-+-+-.
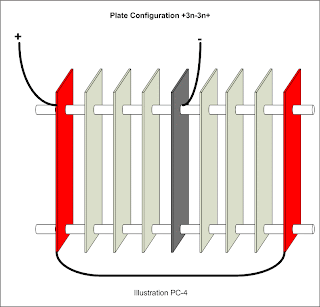
A fourth design with the negative plate in the center and positive plates on either end would look like this: +nnn-nnn+. The only difference from one variation to another would be the number of neutral plates.
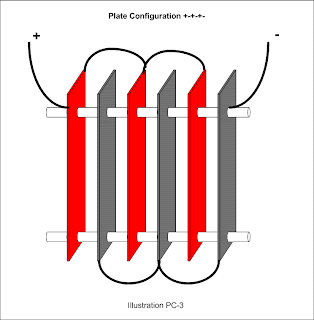
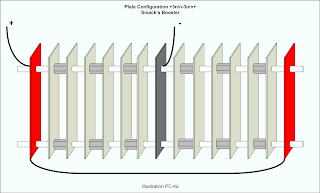
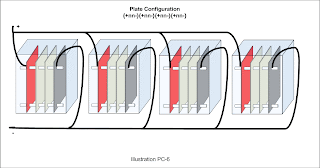
The Smack's Booster design employs neutral plates that are connected electrically. To account for electrically connected neutral plates, use the / character between n's. It would look like this: +3n/n-3n/n+ to designate three pairs of connected neutral plates on either side of the negative plate. To improve readability the same design may include parentheses like so: +3(n/n)-3(n/n)+
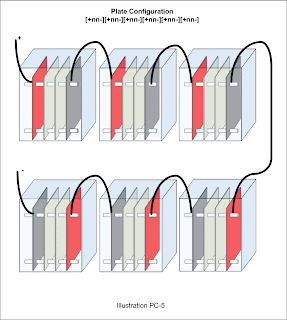
A multi-cell design with 6 cells wired in series may look like so:
[+nn-][+nn-][+nn-][+nn-][+nn-][+nn-]. The same design my be abbreviated thus: 6[+nn-]
A multi-cell design with 4 cells wired in parallel may look like this:
{+nn-}{+nn-}{+nn-}{+nn-} or like this: 4{+nn-}
Pipe and Rod Assemblies
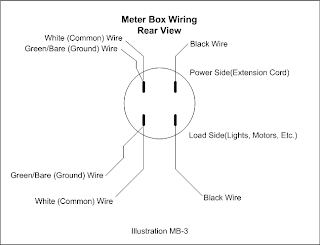
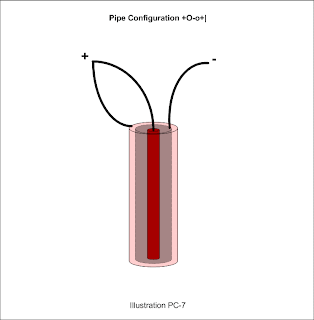
For pipe and rod type configurations, use the Capitol O for a outer pipe, the Small o for an inner pipe and the pipe character | for a rod. To write this nomenclature, first enter the polarity then the characteristic letter and repeat until finished. So a Pipe/Pipe/Rod configuration might be written +O-o+|.
To designate multiples, simply enter the number in the configuration and put the configuration in square braces or parentheses to indicate the connection type, like so 4[+O-o+|] to indicate 4 assemblies wired in series or 4{+O-o+|} to indicate the same but in parallel. If the four assemblies are in the same cell, then it would be written this way: [4(+O-o+|)] although they are more likely in parallel: {4(+O-o+|)}
Another possibility with the Pipe/Pipe/Rod configuration is that of the small pipe being neutral. It might look like so: +Ono-|.

Helical and Spring Designs
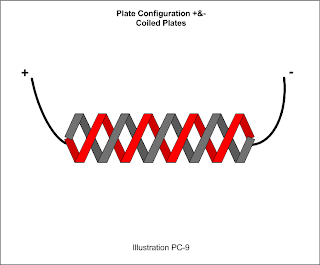
Additionally, for the one helical and one spring design I've seen, I suggest using the Ampersand (&) to indicate such. This example would be +&- to indicate the two plates actually wrap around each other.
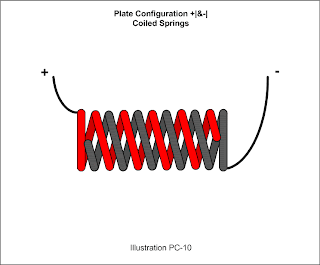
For a spring design, the main difference would be that it is designated as a rod like so: +|&-|
Spiral Designs
A spiral assembly is one where the plates wrap one around the other to make a sort of compact spring. This type would be designated with the @ symbol like this: +@-.
Conclusion
From this a large variety of electrode combinations and designs can be represented; thus anyone should to be able to replicate a design from the plate configuration nomenclature and the plate dimension specifications. If I've missed something, please let me know.
Glossary
Polarity Designations
+ : Positive Electrode
- : Negative Electrode
n : Neutral Electrode
Wiring Designations
/ : Electrically Connected Plates(usually with a metallic nut, washers or spacers)
[] : Series wiring Configuration
{} : Parallel wiring Configuration
Shape Designations
P : Plate, optional
B : Bowled modifier for plate
C : Conical modifier for plate
O : Outer Pipe
o : Inner Pipe
| : Rod
& : Spring or Helix depending on rod or plate electrode
@ : Spiral plates
Multiplier and Grouping Designations
1-99 : The number of times to repeat the following designation.
() : Designation grouping. Used for improved readability.
[] : Series wiring Configuration (Indicates everything inside the braces are in a single cell)
{} : Parallel wiring Configuration (indicates everything inside the parentheses are inside a single cell)
Documentation & Terminology Articles
Next:
Proposed Electrolysis Supplementation System Block Diagram Previous:
What on Earth am I Doing?